Part 1 of the 3-part series discussed the development work behind isolating antigens in K9-ACV and the background of Dr. John R. Yannelli. In part 2 we look at what drives the immune learning response of an immunotherapy vaccine – the adjuvant designed by Dr. Donald Cohen.
Adjuvants
An adjuvant is a substance that enhances the body’s immune response to an antigen. Adjuvants are designed to be strong enough to trigger a safe and effective immune response. Their effectiveness may also control vaccine costs by reducing primary ingredient thresholds.

Donald Cohen, PhD
Dr. Donald Cohen is Professor Emeritus at the University of Kentucky College of Medicine and the founding Director of the Core Flow Cytometry Department. Dr. Cohen is the inventor of the Ardent canine adjuvant utilized in K9-ACV. Dr. Cohen earned his Doctor of Philosophy in Microbiology from the University of Cincinnati, OH, in 1979 and completed his Post Doctoral training as a research fellow in cancer immunology for the Virginia Commonwealth University Medical College of Virginia in Microbiology/Immunology from 1979 through February 1982. Here, Dr. Don Cohen first met Dr. John Yannelli. These two cancer researchers would team up to create K9-ACV nearly four decades later.
In 1986 Dr. Cohen became a professor at the University of Kentucky College of Medicine, becoming Professor Emeritus in 2022. In 1996, Dr. Cohen became the Scientific Director of the Flow Cytometry Facility. Dr. Cohen was instrumental in assisting the University gain National Cancer Institute (NCI) designated status for its Markey Center. If you ask Dr. Cohen about his hundreds of publications and accomplishments, the success of his graduate & post-doctoral trainees are among his most fond accomplishments.
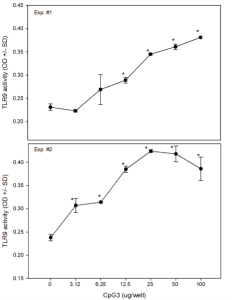
Activation of intracellular TLR9 by canine-optimized CpG ODN. The HEK-Blue™ hTLR9 reporter cell line was cultured in HEK-Blue™ medium in the presence of an increasing concentration of the canine-optimized CpG ODN, CpG3. Following overnight culture, the level of SEAP in culture supernatants was measured in a spectrophotometer at 620-655 nm. SEAP activity is presented as O.D. + S.D. of triplicate wells.
CpG Adjuvants
Cytosine phospoguanine, or CpG, is a synthetic form of DNA that mimics bacterial and viral genetic material. CpG mimics a pattern of DNA common in bacteria and viruses but rarely found in vertebrates. CpG oligodeoxynucleotides (ODNs) bind to and activate Toll-like receptor 9 (TLR9), initiating an innate immune response that supports the subsequent development of adaptive immunity. Pre-clinical studies demonstrate that TLR9 agonists improve antigen presentation and induction of vaccine-specific cellular and humoral responses. Over 100 clinical trials utilizing CpG ODNs have been conducted that evaluated their utility in preventing or treating allergies, infectious diseases, and cancer[1].
For the Dog! “In Silico” Designed, “In Vitro” Tested, and “In Vivo” validated.
For precision medicine to be effective, one must consider the species target. K9-ACV was developed specifically for dogs with cancer. The CpG ODN adjuvant was designed “in silico”, which is modern terminology to describe experimentation performed by a computer algorithm. This in-silico-developed adjuvant was then tested “in vitro” or in a petri dish.
An important feature of whole tumor cell vaccines is the expression of immunogenic neoantigens, mutated normal cell proteins that uniquely express in each individual with a tumor. A cost-effective approach is the development of whole tumor cell autologous vaccines that can elicit immune responses in canine patients against all neoantigens expressed in their own tumors. While exposure to tumor neoantigens can stimulate the immune response in tumor-bearing animals, elicitation of an effective anti-tumor response typically requires additional stimulation of the immune system by inclusion of adjuvants that mimic bacterial and viral molecules that signal the immune system to the presence of pathogens, so-called pathogen-associated molecular patterns (PAMPS). The inclusion of a CpG oligodeoxynucleotide to dead tumor cells in our autologous cancer vaccine acts as a PAMP to elicit anti-tumor immunity against deceased tumor cells effectively. Importantly, the process by which tumor cells are killed in vaccine preparation can further improve the development of anti-tumor immune responses.
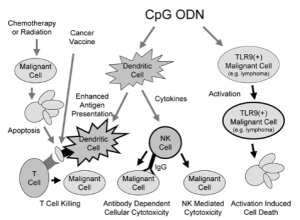
CpG ODN Flowchart
Dr. Cohen demonstrated that the canine-optimized CpG ODN used in the autologous tumor vaccine effectively activates intracellular TLR9 and is superior to commercially available CpG ODNs in stimulating canine dendritic cells to secrete IL-12, which is critical for the development of an effective cellular immune response to tumor antigens. CpG also activates dendritic cells, promoting interactions with T cells to elicit anti-tumor immune responses.
The last process of the adjuvant design, which is proprietary to this specific sequenced product for dogs, was to design it in a way that could deliver a dual “adjuvant mechanism of action”. In this case, the adjuvant was designed as an immune potentiator, as described above, and a delivery system, which is not described in this article. The latter was a design addition added to the sequence to enhance vaccine effectiveness.
The vaccine adjuvant in K9-ACV has been scientifically developed to achieve the best results in canine patients.
[1] https://www.sciencedirect.com/science/article/abs/pii/B9780128040195000098?via%3Dihub#preview-section-abstract

Recent Comments